Thrips parvispinus (Karny, 1922)
Thripinae, Thripidae, Terebrantia, Thysanoptera
Figures
Fig. 1: 7-segmented antenna, segments III and IV with forked sense cone, terminal segments V-VII
Fig. 2: Head dorsal with ocellar triangle
Fig. 3: Pronotum
Fig. 4: Meso- and metanotum
Fig. 5: Fore wing and fore wing middle region
Fig. 6: Sternites VI and VII
Fig. 7: Tergites VI and VII
Fig. 8: Tergites VII and VIII
Fig. 9: Tergites VIII and IX
Introduction and recognition
Thrips parvispinus is a polyphagous thrips breeding in flowers and on young leaves of papaya, pepper and potato, and are key pest of Gardenia plants. Both sexes fully winged. Body brown; head and thorax paler than abdomen; head commonly with cheeks darker than median area; legs mainly yellow; antennal segment III yellow, also basal half of IV & V; fore wings brown with base sharply pale. Antennae 7-segmented; segments III & IV elongate with apex constricted and forked sense cone (Fig. 1). Head wider than long; with 2 pairs of ocellar setae, pair I absent, pair III small and arising on anterior margins of ocellar triangle; postocular setae I & III about as long as ocellar setae III, postocular setae II minute (Fig. 2). Pronotum with 2 pairs of long posteroangular setae; posterior and anterior margin each with 3-4 pairs of setae (Fig. 3). Mesofurca with spinula. Metanotum with mainly equiangular reticulation, reticles varying in shape and sometimes with faint internal sculptured markings; median setae longer than lateral setae and arising well behind anterior margin; campaniform sensilla absent (Fig. 4). Mid and hind tarsi 2-segmented. Fore wing first and second vein with complete rows of setae; clavus with 5 marginal setae, clavus terminal seta longer than subterminal seta (Fig. 5). Tergite II with 3 or 4 lateral marginal setae; V-VIII with ctenidia present laterally, on VIII posteromedial to spiracles; posterior margin of VIII with comb almost absent, a few microtrichia present laterally (Fig. 7-9); pleurotergites without discal setae. Sternite II with 2 pairs of marginal setae, III-VII with 3 pairs, median marginal setae on VII arising in front of posterior margin; sternites II & VII without discal setae, III-VI with 6-13 discal setae in an irregular row (Fig. 6).
Male largely yellow; tergite VIII with no posteromarginal comb; tergite IX setae S1 slightly longer than S2, bases equidistant; sternites III-VII each with small transverse glandular area, discal setae arising laterally.
Taxonomic identity
Species
Thrips parvispinus (Karny, 1922)
Taxonomic history
Thrips (Isoneurothrips) taiwanus Takahashi, 1936
Isoneurothrips pallipes Moulton, 1928
Isoneurothrips jenseni Karny, 1925
Isoneurothrips parvispinus Karny, 1922
Common name
Taiwanese thrips
Present taxonomic position
Family: Thripidae Stephens, 1829
Subfamily: Thripinae (Stephens) Karny, 1921
Genus: Thrips Linneaeus, 1758
Genus
The genus Thrips L., 1758
There are nearly 300 species currently recognized in the genus Thrips making this genus one of the largest taxa within the order Thysanoptera. The genus was redefined progressively during the 1970's (see Mound et al. 1976), to include many species previously placed in Taeniothrips. The genus Thrips now includes a range of species, some with the antennae 7-segmented, others 8-segmented, and a few with the number of segments varying between 7 and 8. Similarly, some species have few setae on the fore wing first vein, whereas others have a complete row of setae on this vein. The species with a complete setal row on the first vein were placed from some taxonomists in the genera Isothrips or Isoneurothrips. However, all of the species in Thrips have the following character states: antennal segments III & IV with forked sense cone, absence of ocellar setae I, pronotum with 2 pairs of elongate posteroangular setae, paired ctenidia laterally on the tergites V-VIII, tergite VIII ctenidium arising posterior to the spiracle (in contrast to species of the genus Frankliniella). Other character states, such as number of antennal segments, number of setae on the fore wing veins, and number of discal setae on the abdominal sternites are variable between species (Mound & Masumoto 2005; Nakahara 1994; Palmer 1992). Identification keys are available for the species of this genus from many parts of the world. Of particular importance is the published key by Mound (2010) for members of the genus Thrips from Afro-tropical region as well as previous Lucid keys from Moritz et al. (2001, 2004, 2009).
Species description
Typical key character states of Thrips parvispinus
Coloration and body sculpture
Body color: mainly brown to dark brown
Surface of head, pronotum and fore legs: without obvious or with weakly reticulate sculpture
Antennae
Number of antennal segments: 7
Antennal segment I: without any setae on dorsal apical margin
Antennal segment II: without an exceptionally long seta at the inner apex
Antennal segment II shape: symmetric
Antennal segment III shape: symmetric
Length of antennal segment III and IV: antennal segment III similar in length to segment IV
Form of sense cones on antennal segments III and IV: emergent and forked on segments III and IV
Antennal segment IV and V: without a hyaline ring near the base
Forked sense cone on antennal segment IV: scarcely extending beyond base of segment V
Antennal segment VI bears: not a remarkably dagger-shaped sensorium
Head
Distance between bases of ocellar setae III: greater than width of first ocellus
Head: not prolonged in front of compound eyes
Ocellar setae I: absent
Length of ocellar setae II: shorter than setae III
Ocellar setae III: arising on anterior margin of, or in front of ocellar triangle
Ocelli: present
Length of postocular setae: not alternating short and long setae
Number of ocellar setae: 2
Prothorax
Number of pairs of anteromarginal minor setae: 3-4
Number of pairs of long anteroangular setae: 0
Number of pairs of long posteroangular setae: 2
Number of pairs of elongate pronotal setae: 2
Number of pairs of posteromarginal minor setae: 3-4
Pronotal blotch or internal apodeme: absent
Pronotum shape: broadly rectangular
Pronotum posteromarginal/posteroangular setae: S2 longer than S3, not equal in length
Mesothorax
Mesosternal furca: with median spinula
Metathorax
Metanotal campaniform sensilla: absent
Reticulations of metanotal sculpture: rarely with faint internal sculptured markings
Metanotal median setae: S1 behind anterior margin
Metanotum with dominant sculptured triangle medially: absent
Metasternal furca: without spinula
Sculpture of metanotum median area: with mainly equiangular reticulation
Shape of metathoracic furca: transverse, V-shaped
Metanotal median setae length: longer than lateral metanotal setae
Wings
Fringe cilia arising: from sockets
Fore wing veins: present
Fore- and hind wing surface: covered with microtrichia
Fore and hind wings: present, more than half as long as abdomen (macropterous)
Apex of fore wing: with prominent terminal setae
Fore wing anterior margin (costal vein): with setae and cilia but cilia longer than setae
Fore wing clavus - number of marginal setae: 5
Fore wing clavus - terminal veinal seta: longer than subterminal seta
Fore wing costal fringe cilia: arising at anterior margin of wing
Fore wing first vein: distinct from costal vein
Fore wing first vein setal row: complete, with setae closely and uniformly spaced
Fore wing second vein setal row: complete, setae uniformly spaced
Fore wing shape: mainly parallel sided or margins run continuously towards each other
Fore wing surface: not reticulate
Fringe cilia on posterior margin near apex: distinctly wavy (undulated)
Length of fore wing costal setae at middle of wing: longer than half of median wing width
Shape of fore wing apex: with mainly posterior margin curved to join anterior margin
Fore wing extreme apex color: dark
Fore wings: uniformly dark or shaded, but with base or sub-base pale
Legs
Fore tibia: not prolonged around fore tarsus
Mid and hind tarsi: with two segments
Color of fore tarsi: pale or yellow, sometimes apical shaded or brown
Abdomen
Pleurotergal discal setae: absent
Pleurotergites: not covered in microtrichia
Number of pleurotergal discal setae: 0
Sternite II: with marginal setae but no discal setae
Number of discal setae on sternites III to VI: 6-12 (13)
Sternites IV, V and VI: with marginal setae and discal setae medially
Pairs of posteromarginal setae on sternites V and VI: 3
Sternite VII median posteromarginal setae S1: arising in front of posterior margin
Sternite VII: with marginal setae but no discal setae
Surface of lateral thirds of abdominal tergites: without regular rows of fine microtrichia
Number of lateral marginal setae on tergite II: 3-4
Sculpture of tergites II to VIII: with one or without transverse lines of sculpture between median pair of setae S1
Tergites II to VII median setal pair: no more than 0.3 as long as median length of tergite
Craspedum on tergites IV to VI: absent
Tergites IV and V median setal pair: shorter than distance between their bases
Markings on tergites IV to VI: without shaded areas medially
Tergites V to VII: with ctenidia laterally
Craspedum on tergite VIII: without craspedum medially and toothlike microtrichia laterally
Tergite VIII ctenidia: posteromedial to spiracle
Color of tergites IX and X: dark or brown
Tergite X: not tubular, longitudinally incomplete
Setae on abdominal tergite X: all setae slender

Similar or related species
Thrips parvispinus is very similar to some other Thrips species - like Thrips orientalis. Thrips parvispinus has discal setae on sternites III-VI but not on sternite VII, and no discal setae on pleurotergites (Thrips australis, Thrips microchaetus, Thrips subnudula and Thrips tenellus, all of them have sternites III-VII with at least 1 pair of discal setae and pleurotergites with discal setae; Thrips acaciae, Thrips brevisetosus, Thrips florum, Thrips gowdeyi, Thrips hawaiiensis and Thrips simplex, all of them have sternites III-VII with at least 1 pair of discal setae and pleurotergites without discal setae; Thrips nigropilosus, Thrips palmi, Thrips pusillus and Thrips tabaci, all of them have sternites and pleurotergites without discal setae).
Thrips parvispinus differs from Thrips orientalis in having sternites III-VI with 6-13 discal setae in an irregular row, metanotal equiangular reticulations rarely with faint internal sculptured markings, brown fore wings with base sharply paler, on fore wing clavus the terminal veinal seta is longer than subterminal seta, and the fore wing first vein setal row is complete. Thrips orientalis has sternites III-VI with no more than 6 discal setae placed laterally, metanotal equiangular reticulations with many conspicuous internal sculptured markings, brown fore wings only with small white spot near veinal fork, on fore wing clavus the terminal veinal seta is shorter than subterminal seta, and the fore wing first vein is highly variable on distal half, from almost complete to only 2 setae medially and 4 distally. Outside this group, the species is similar to Thrips australis in having a metanotal median sculpture with mainly equiangular reticulation, the fore wing first vein with setal row complete, and the comb of tergite VIII is present laterally and absent medially.
Species of the genus Thrips are similar to species of Stenchaetothrips, Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata because of tergites V-VIII bear a pair of ctenidia laterally, which placed on tergite VIII posteromedial to the spiracle, and all species have no ocellar setae I. In contrast to species with craspedum on tergites II-VII (Microcephalothrips abdominalis, Larothrips dentipes and Fulmekiola serrata), species of Thrips and Stenchaetothrips have no posteromarginal craspedum on tergites and sternites. Species of the genus Thrips as well as Fulmekiola serrata and species of Stenchaetothrips have 2 pairs of elongate posteroangular setae (Microcephalothrips abdominalis with 2 pairs of moderately elongate pronotal setae and Larothrips dentipes without elongate setae). Compared to the species of Thrips, Microcephalothrips abdominalis, and Larothrips dentipes, which have ocellar setae II on head much shorter than or about as long as III, Fulmekiola serrata and species of Stenchaetothrips have ocellar setae II much longer than III, and sternites always without discal setae.
Biology
Life history
As with other thrips species the life cycle from egg to adult is dependent on temperature. The full cycle can take about 15 days (Lewis 1973) to over a month. Adults may live for more than one month producing several generations in one year depending on seasons. The development period ranged from 18.8 days at 30°C to 37.6 days at 20°C and the adult fecundity ranged from 50-69 eggs per female (Murai et al. 2010).
Host plants
Polyphagous. Black Jack (Bidens pilosa), coffee, Gardenia sp., papaya, chilli pepper, paprika, potato, tobacco, Vigna sp., green bean, strawberry, eggplant, watermelon and other Cucurbitaceae.
Vector capacity
Tobacco streak virus (TSV). Mechanical transmission of Cladosporium oxysporum causing bunchy-top, i. e. malformed leaves and shot holes on new flushes (Lim 1989).
Damage and symptoms
This polyphagous species has been recorded as a serious pest of Gardenia and as a moderate pest on several of its host plants as a result from the damage caused by feeding and breeding on the young leaves and flowers. Infested leaves lightly mottled and streaked. Recently Thrips parvispinus has been observed as key pest of chilli pepper and strawberry. In Indonesia it has replaced Thrips palmi as the key thrips on vegetables (Murai et al. 2010).
Detection and control strategies
Thrips parvispinus are attracted to white rather than blue or yellow (Murai et al. 2010). Chilli pepper lines resistant to Thrips parvispinus have been identified (Maharijaya et al. 2011). Laboratory studies indicated that they are susceptible to spinosad and not to acetamiprid (Murai et al. 2010). Ladybird beetles, Menochilus sexmaculatus and Coccinella transversalis were observed as potential natural enemies of this species in Indonesia. Entomopathogenic fungus Verticillium lecanii (=Lecanicillium longisporum) and Beauveria bassiana are reported to be effective for against Thrips parvispinus management. Use of Menochilus sexmaculatus and entomopathogenic fungus, Verticillium lecanii (=Lecanicillium longisporum) was as effective as pesticide application for management of Thrips parvispinus (Prabaningrum et al. 2006).
Exposure to 60% CO2 atmospheres at 30°C results in 100% mortality of five different thrips species, Frankliniella occidentalis (Pergande), Frankliniella intonsa (Trybom), Thrips tabaci Lindeman, Thrips palmi Karny, and Thrips parvispinus Karny (Seki & Murai 2012).
Additional notes
-
Biogeography
Asia, Australia, New Zealand, Europe, Africa. La Réunion, Tanzania (Dar-el-Salaam), Uganda (Kampala).
African countries where Thrips parvispinus has been reported
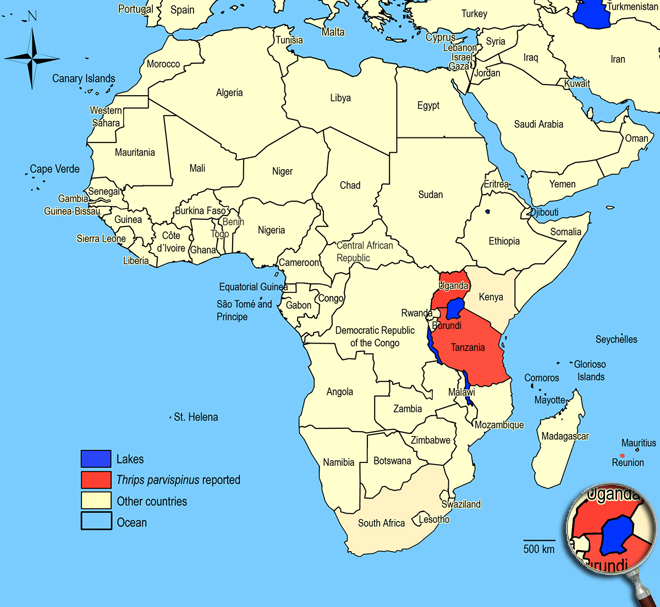
Occurence of Thrips parvispinus in East Africa
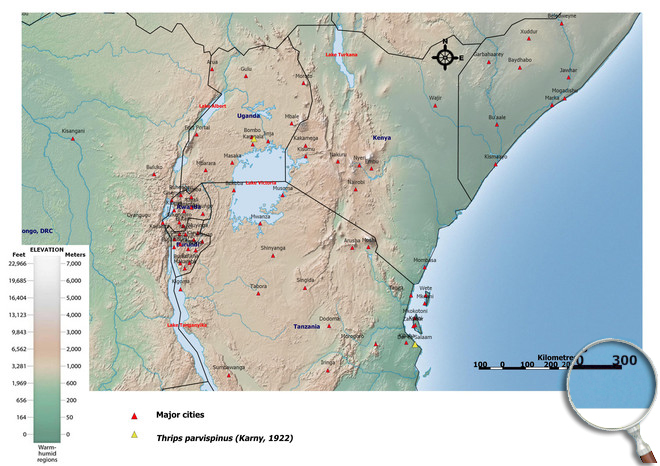
Please click here for survey sites of all observed thrips species of Kenya, Tanzania and Uganda.
Click here for locations of Thrips parvispinus in parts of East Africa.

Bibliography
Bournier J-P (2000). Les thysanoptères de l´île de la Réunion: Terebrantia. Bulletin de la Société Entomologique de France. 105 (1): 65-108
Jones DR (2005). Plant viruses transmitted by thrips. European Journal of Plant Pathology. 113: 119-157
Karny H (1922). Thysanoptera from Siam and Indo-China. Journal of the Siam Society. 16: 91-153
Karny H (1925). Die an Tabak auf Java und Sumatra angetroffenen Blasenfüsser. Bulletin van het deli Proefstation te Medan. 23: 1-55
Klose MJ, Sdoodee R, Teakle DS, Milne JR, Greber RS & Walter GH (1996). Transmission of three strains of tobacco streak ilarvirus by different thrips species using virus-infected pollen. Journal of Phytopathology. 144 (6): 281-284
Lewis T (1973). Thrips: their biology, ecology and economic importance. Academic Press Inc., London Ltd., 349 pp
Lewis T (1997). Thrips as crop pests. CAB International, Wallingford, 740 pp
Lim WH (1989). Bunchy and malformed top of papaya cv. Eksotika caused by Thrips parvispinus and Cladoaporium oxysporum. Jurnal penyelidikan Mardi (MARDI Research Journal). 17: 200-207
Maharijaya A, Vosman B, Steenhuis-Broers G, Harpenas A, Purwito A, Visser RGF & Voorrips RE (2011). Screening of pepper accessions for resistance against two thrips species (Frankliniella occidentalis and Thrips parvispinus), Euphytica 177:401-410
Moritz G (2006). Thripse. Pflanzensaftsaugende Insekten, Bd. 1, (1. Auflage). Westarp, Hohenwarsleben, 384 pp. ISBN-13: 978 3 89432 891 7
Moritz G, Morris DC & Mound LA (2001). ThripsID - Pest thrips of the world. ACIAR and CSIRO Publishing Collingwood, Victoria, Australia, CDROM ISBN 1 86320 296 X
Moritz G, Mound LA, Morris DC & Goldarazena A (2004). Pest thrips of the world - an identification and information system using molecular and microscopical methods. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN 1 86499 781 8
Moritz G, O'Donnell C & Parrella M (2009). Pest thrips of North America. Centre for Biological Information Technology, University of Queensland, Australia, CDROM ISBN-13: 978 1 86499 940 2
Moulton D (1928). Thysanoptera of Japan: New species, notes, and a list of all known Japanese species. Annotationes Zoologicae Japonenses. 11 (4): 287-337
Mound LA (2010). Species of the genus Thrips (Thysanoptera, Thripidae) from the Afro-tropical Region. Zootaxa. 2423: 1-24
Mound LA & Collins DW (2000). A south east Asian pest species newly recorded from Europe: Thrips parvispinus (Thysanoptera: Thripidae), its confused identity and potential quarantine significance. European Journal of Entomology. 97: 197-200
Mound LA & Kibby G (1998). Thysanoptera: An identification guide, (2nd edition). CAB International, Wallingford and New York, 70 pp
Mound LA & Masumoto M (2005). The genus Thrips (Thysanoptera, Thripidae) in Australia, New Caledonia and New Zealand. Zootaxa. 1020: 1-64
Mound LA, Morison GD, Pitkin BR & Palmer JM (1976). Thysanoptera. Handbooks for the identification of British insects, Vol. 1, Part 11. Royal Entomological Society of London, London, 79 pp
Murai T, Watanabe H, Wataru T, Adati T & Okajima S (2010). Damage to vegetable crops by Thrips parvispinus Karny (Thysanoptera: Thripidae) and preliminary studies on biology and control. In: Persley D, Wilson C, Thomas J, Sharman M, Tree D. (eds). IXth International Symposium on Thysanoptera and Tospoviruses, 31 August- 4 September, 2009. Journal of Insect Science 10:166 available online: insectscience.org/10.166
Nakahara S (1994). The genus Thrips Linnaeus (Thysanoptera: Thripidae) of the New World. Technical Bulletin, USDA, Agricultural Research Service. 1822: 1-183
Palmer JM (1992). Thrips (Thysanoptera) from Pakistan to the Pacific: a review. Bulletin of the British Museum (Natural History), Entomology. 61 (1): 1-76
Palmer JM, Mound LA & du Heaume GJ (1989). 2. Thysanoptera, 73 pp. In Betts CR [ed.], CIE Guides to insects of importance to man. CAB International, Wallingford, Oxon, UK
Potts MJ & Gunadi N (1991). The influence of intercropping with Allium on some insect populations in potato (Solanum tuberosum). Annals of Applied Biology. 119 (1): 207-213
Prabaningrum, L., Moekasan, T.K., Udiarto, B.K., den Belder, E. & Elings, A. (2008). Integrated Pest Management on Sweet pepper in Indonesia: Biological control and control thresholds for thrips. Acta Horticulturae (ISHS) 767:201-210, http://www.actahort.org/books/767/767_20.htm
Priesner H (1934). Indomalayische Thysanopteren (VI). Natuurkundig Tijdschrift voor Nederlandsch-Indie. 94 (3): 254-290
Reyes CP (1994). Thysanoptera (Hexapoda) of the Philippine Islands. The Raffles Bulletin of Zoology. 42 (2): 107-507
Seki M & Murai T (2012). Responses of five adult thrips species (Thysanoptera; Thripidae) to high-carbon dioxide atmospheres at different temperatures. Applied Entomology and Zoology 47(2):125-128
Vos JGM & Nurtika N (1995). Transplant production techniques in integrated crop management of hot pepper (Capsicum spp.) under tropical lowland conditions. Crop Protection. 14 (6): 453-459
----
Web links
Mound´s Thysanoptera pages
Thysanoptera Checklist
ICIPE Thrips survey sites
UNI Halle & Thrips sites
Thrips of California













